[ad_1]

Chemical engineers at the Massachusetts Institute of Technology (MIT) have devised an efficient way to convert carbon dioxide into carbon monoxide, using a DNA-linked catalytic process that could significantly reduce greenhouse gas emissions. This achievement offers a new path to producing valuable chemicals from carbon dioxide, with the potential for large-scale industrial application. Credit: SciTechDaily.com
Catalyst tethered to it DNA It enhances the efficiency of the electrochemical conversion of carbon dioxide to carbon dioxide, and is a building block for many chemical compounds.
Massachusetts Institute of Technology Chemical engineers have devised an efficient way to convert carbon dioxide into carbon monoxide, a chemical that can be used to generate useful compounds such as ethanol and other fuels.
If the process is scaled up for industrial use, it could help remove carbon dioxide from power plants and other sources, reducing the amount of greenhouse gases released into the atmosphere.
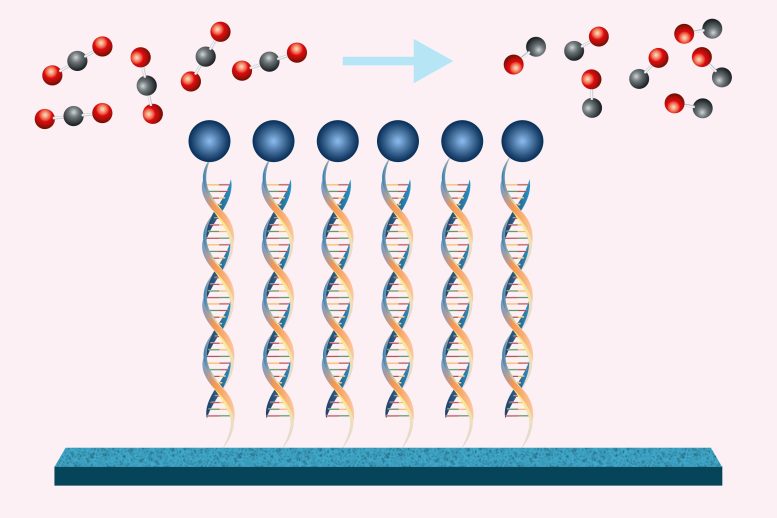
Chemical engineers at MIT have shown that by using DNA to attach a catalyst (blue circles) to an electrode, they can make the conversion of carbon dioxide to carbon monoxide more efficient. Credit: Christine Daniloff, MIT; iStock
Revolutionary carbon removal technology
“This will allow you to take carbon dioxide from emissions or dissolved in the ocean, and turn it into profitable chemicals. It's really a way forward for decarbonization because we can get rid of carbon dioxide2“It's a greenhouse gas, and turning it into things that are useful for chemical manufacturing,” says Ariel Forrest, the Paul M. Cook Career Development Assistant Professor of Chemical Engineering and lead author of the study.
The new approach uses electricity to perform the chemical conversion, with the help of a catalyst attached to the electrode surface by DNA strands. This DNA acts like Velcro to keep all the components of the reaction close together, making the reaction more efficient than if all the components were floating in the solution.
Forrest created a company called Helix Carbon to further develop the technology. Gang Fan, a former researcher at the Massachusetts Institute of Technology (MIT), is the lead author of this paper, which appears in the MIT Journal Journal of the American Chemical Society Au. Other authors include Nathan Corbin PhD ’21, Minju Zhong PhD ’23, and former MIT postdoctoral researchers Thomas Gill and Amruta Karbalkar, and Evan Moore ’23.
Breaking a company2
Converting carbon dioxide into useful products first requires converting it into carbon monoxide. One way to do this is by using electricity, but the amount of energy required for this type of electrical stimulation is expensive.
To try to reduce these costs, researchers have tried using electrocatalysts, which can speed up the reaction and reduce the amount of energy that must be added to the system. One type of catalyst used in this reaction is a class of molecules known as porphyrins, which contain metals such as iron or cobalt and are similar in structure to the heme molecules that carry oxygen in the blood.
During this type of electrochemical reaction, carbon dioxide is dissolved in water inside an electrochemical device, which contains an electrode that drives the reaction. The catalysts are also suspended in solution. However, this setup is not very efficient because the carbon dioxide and catalysts have to meet each other on the electrode surface, which does not happen often.
To make the reaction occur repeatedly, which would enhance the efficiency of electrochemical conversion, Forrest began working on ways to attach catalysts to the electrode surface. DNA appears to be the perfect choice for this application.
“DNA is relatively inexpensive, you can modify it chemically, and you can control the interaction between two strands by changing the sequences,” she says. “It's like the Velcro of a sequence that has very strong interactions but they're reversible and you can control them.”
To attach single strands of DNA to a carbon electrode, the researchers used two “chemical handles,” one on the DNA and one on the electrode. These handles can be linked together to form a permanent bond. The complementary DNA sequence is then attached to the porphyrin catalyst, such that when the catalyst is added to the solution, it will bind reversibly to the DNA already attached to the electrode – just like Velcro.
Once this system is set up, researchers apply a voltage (or bias) to the electrode, and the catalyst uses this energy to convert carbon dioxide in the solution into carbon monoxide. The reaction also generates a small amount of hydrogen gas from the water. After the catalysts are eroded, they can be released from the surface by heating the system to break the reversible bonds between the two DNA strands, replacing them with new ones.
Pioneering electrochemical conversion
Using this approach, the researchers were able to boost the Faradaic efficiency of the reaction to 100%, meaning that all the electrical energy entering the system goes directly to the chemical reactions, without wasting any energy. When the catalysts are not bound to DNA, Faradaic efficiency is only about 40 percent.
This technology can be scaled up for industrial use fairly easily, Forrest says, because the carbon electrodes the researchers used are much less expensive than traditional metal electrodes. Catalysts are also inexpensive, because they do not contain any precious metals, and only a small concentration of catalyst is needed on the electrode surface.
By swapping out different catalysts, the researchers plan to try making other products such as methanol and ethanol using this approach. Helix Carbon, founded by Forrest, is also working to further develop the technology for potential commercial use.
Reference: “Highly Efficient Electroreduction of Carbon Dioxide via DNA-Directed Catalyst Immobilization” by Gang Fan, Nathan Corbin, Mingyu Zhong, and Thomas M. Gill, and Evan B. Moore, and Amruta A. Karpilkar, and Ariel L. Forrest, March 25, 2024, JAX AU.
doi: 10.1021/jacsau.3c00823
The research was funded by the U.S. Army Research Office, the CIFAR Azrieli Global Scholars Program, the MIT Energy Initiative, and the MIT Deshpande Center.
[ad_2]
Source

